|  |
JHY Services & Support
FAQs & Tips
Since its introduction in early 1989, there has been significant interest among the dental profession and the general public for home-use tooth bleaching products and methods. Typical dental bleaching compositions include from 5-20% by weight of carbamide peroxide (CO(NH2)2.H2O2), which is a complex of urea and hydrogen peroxide. However, sodium perborate has been found to be an another dental bleaching agent. An advantage of perborate-based bleaching agents rather than aqueous hydrogen peroxide or carbamide peroxide is that perborates are allowed for dental bleaching procedures in some countries that do not permit the use of aqueous hydrogen peroxide and carbamide peroxide for dental bleaching. Perhaps perborate compounds are more gentle on surrounding gums and tissues compared to either aqueous hydrogen peroxide or carbamide peroxide. Nevertheless, perborates were found to be unstable when blended with carboxypolymethylene, which is the tackifying agent of choice in the vast majority of home bleaching kits presently on the market. For this reason, a tackifying agent that is stable in the presence of perborate bleaching agents has been developed, which comprises a mixture of a suitable polyol and a finely divided gel-forming particulate such as fumed silica, otherwise known as silica fume.
Below is a sample dental bleaching composition that combines the following ingredients (in weight percent):
*Anhydrous Propylene Glycol 54.3%
*Fumed Silica 20%
*Sodium Perborate Monohydrate 25%
*Sodium Saccharine 0.7%
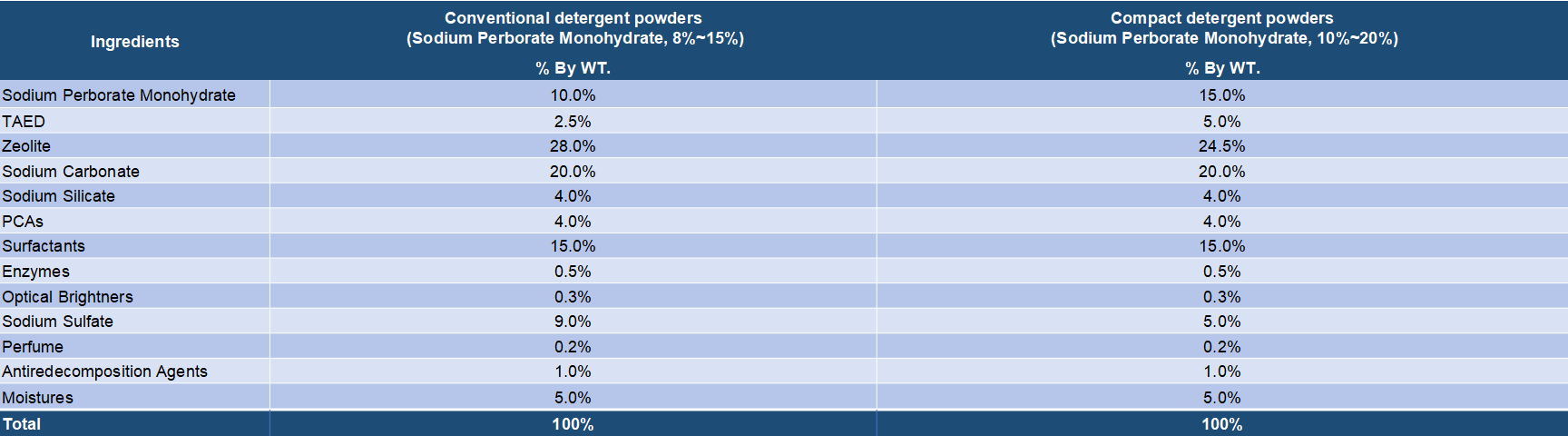
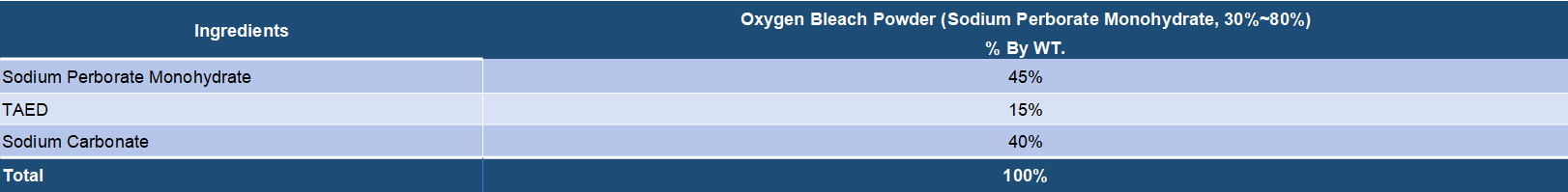
Both sodium perborate and sodium percarbonate are oxygen release bleaching chemicals that are widely applied in various bleach compositions. It is known that sodium perborate as the bleaching agent has a high bleaching effect at high temperatures but the effect is lowered at low temperatures. On the other hand, sodium percarbonate has an effective bleaching action even at low temperatures and is very valuable from the viewpoint of saving of energy. Sodium percarbonate is an attractive perhydrate for use in detergent compositions because it dissolves readily in water, is weight efficient and, after giving up its available oxygen, provides a useful source of carbonate ions for detergency purposes. Sodium serborate has a better stability and has been a mature bleaching ingredients for long time. But it is increasingly replaced by sodium percarbonate duo to its disadvantages in energy saving and environment protection. Sodium percarbonate exhibits an excellent bleaching effect even at a low temperature and is environmentally friendly, but it is less stable in detergent formulations. However, many processes have been found to improve its stability.
Sodium perborate usually exists in two forms, tetrahydrated and monohydrated. Sodium perborate tetrahydrate is obtained by addition of hydrogen peroxide to a sodium metaborate solution at a temperature close to 20.degree. C. Sodium perborate monohydrate is produced by dehydrating sodium perborate tetrahydrate in a fluid bed with heated air. Sodium perborate releases nascent oxygen at elevated temperatures, and so acts as a hydrogen peroxide bleach. The monohydrated form is essentially showing three advantages in comparison with the tetrahydrated form: a higher content of available oxygen, a higher heat stability and a higher dissolution rate into water. Sodium perborate has been in detergent and personal care formulations for many years. Its oxidative power improves the cleaning, bleaching, stain removal and deodorizing performance of powder detergent formulations, all fabric dry bleachs, denture cleaners, automatic dishwasher detergents and various institutional and industrial laundry products. It's main disadvantage is that the bleaching action only takes place at elevated temperatures. To release it's bleaching action at lower temperatures, an activator must be added.








