|  |
JHY Services & Support
FAQs & Tips
Bleaching of pulp for papermaking is roughly divided into bleaching with a peroxide, bleaching with a chlorine-containing oxidizing agent, and bleaching with a reducing agent, and these have been employed depending purpose of use in consideration of their bleaching characteristics and effects. The bleaching with a peroxide represented by on the kind of pulp and H2O2 is employed mainly for bleaching of mechanical pulp and for deinking/bleaching of waste newspaper because of its bleaching effect on pulp containing lignin. Since the bleaching with a chlorine-containing oxidizing agent is effective for chemical pulp containing a slight amount of lignin, bleaching with Cl2, NaCIO or CIO2 is mainly carried out in multistage bleaching of kraft pulp, and bleaching with NaCIO in bleaching of waste wood-free or mechanical paper. For example, in the bleaching of waste wood-free or mechanical paper, a 12% NaCIO solution is added to pulp in an amount of about 8% based on the amount of pulp and mixed therewith, whereby the bleaching is carried out. The bleaching with a reducing agent is excellent in decolonization of dye type coloring materials, but it has a limited- bleaching capability for peroxide type chemicals and chlorine-containing chemicals and entails a high cost. For these reasons and the like, it has been employed mainly for bleaching of mechanical pulp demanding a low brightness grade to be incorporated into newspaper, post-bleaching after H2O2 bleaching of mechanical pulp demanding a high brightness grade and post-bleaching after H2O2 deinking/bleaching of waste newspaper. In general, Na2S2O4 or TUDO of the dithionite family has been used as the reducing agent. Of these, Na2S2O4 has been mainly used from the viewpoint of bleaching capability, chemicals cost, etc. However, TUDO bleaching has recently come to be spotlighted as a substitute for NaCIO bleaching in bleaching of waste wood-free or mechanical paper and as a substitute for Na2S2O4 bleaching in post-bleaching after H2O2 deinking/bleaching of waste newspaper, from the viewpoint of the problem of waste water pollution by organic chlorine compounds represented by dioxins, demand for energy conservation, decoloring effect on dye type coloring materials,etc.
From its discovery in the late 18th century, the history of hydrogen peroxide is over 200 years. Hydrogen peroxide is a highly reactive, strong oxidizing. It can be involved in most of the redox reactions of harmful substances with almost no toxicity and is more biodegradable. Solid hydrogen peroxide has been widely recognized in aquaculture due to its ability to reduce the subaqueous content of ammonium and nitrogen, transfer electrons to dissolved oxygen to form superoxide radicals and increase oxygen levels, prevent anaerobe from proliferation and kill nosogenetic bacteria, and enhance water quality. It is also considered as a treatment against ectoparasites of adult fish, improving fish health in aquaculture to support the sustainability of the aquaculture industry.
Hydrogen peroxide is a chemical compound with the Chemical formula H2O2. Pure hydrogen peroxide is a stable substance kept under optimal conditions for years. However, H2O2 easily decomposes when it is exposed to impurities. Hazard Statements: Hydrogen peroxide may cause fire or explosion, releasing a large amount of heat, oxygen, and water vapor. 2H2O2 → 2H2O + O2 + 196.4 kJ/mol
Oxygen-release compounds increase the oxygen content of contaminated areas, enhancing biological activity and thus promoting natural attenuation. The specific compound used will depend on soil chemistry, concentration of target organics, type of target organics and cleanup levels. Parameters of interest are release rate of oxygen at different effective partial pressures and ratio of oxygen released to amount of oxygen applied. Researchers studied the solid oxidants below with respect to dissolution rate and ease of movement through other media:
- --Na2CO3-1.5H2O2 encapsulated sodium percarbonate
- --Free sodium percarbonate crystals
- --CaO2, calcium peroxide
- --MgO2, magnesium peroxide
Oxygen movement
Oxygen movement in the subsurface is influenced by:
- --Soil heterogeneity
- --Moisture content, which can hinder O2 movement
- --Pore size——a function of sediment age and history
- --Tortuosity, caused by small pore sizes, which increases O2 path distance
Soil morphology directly influences O2 diffusion through the soil and soil redox potential, and the biological degradation that will occur at interfacial areas. In the interstitial pores, microbes are protected from toxic compounds. "Interstitial pore space attachment also makes predation more difficult. Solid oxidants can exhibit slow dissolution and fall into a reaction-limited domain. Conversely, these compounds can release oxygen from their surfaces rapidly, exhibiting transport limitations. Researchers predicted that the encapsulated Na2CO3 +1.5H2O2's release of O2 was by diffusion-limited transport while the other studied oxidants were controlled by chemical reaction kinetics of dissolution. The kinetics of dissolution have both chemical and thermodynamic limitations. Reactions are as follows:
2H2O + MgO2 ↔ Mg(OH)2(s) + H2O2
2H2O + CaO2(s) + ↔ Ca(OH)2(s) + H2O2
4Na2CO3•1.5H2O2 ↔ 8Na+ + 4CO3- + 6H2O2
H2O2 + H2O2 ↔ O2 + 2H2O
Some of the reaction products produced-Mg(OH)2 and Ca(OH)2 -have solubility values lower than the ions added. Such precipitates may coat reactant particles and block pores in both the soil and reactant particles, limiting transport of reacting ions and particles. Sodium percarbonate would release O2 by diffusion-limited transport whereas chemical kinetic reactions would control dissolution rate of other oxidants. Release rates of MgO2 and CaO2 could be limited because of self-encapsulation.
Experiments and results:The unencapsulated Na2CO3• 1.5H2O2 had the most rapid release rate, followed by CaCO2, and encapsulated Na2CO3•1.5H2O2. MgO2 had the slowest O2 release by several orders of magnitude. However, the large size of both forms of Na2CO3-1.5H2O2 slows transport of bulk particles. CaO2 and MgO2 both have fractions small enough to permit migration where soil particles, and thus pore spaces, are larger than the particles of soil oxidant. In some cases, lack of movement of oxidant particles may be desirable in establishing stationary oxidative zones. Adding oxidants to water also changes the water's pH, usually in the range of 10 to 12. Shifts to high pH conditions generally have a negative effect on indigenous bacteria, but soils can have a buffering capacity to counteract or neutralize the pH shifts.
Other conclusions:Release rates that are too rapid for biological uptake rates will prevent the utilization of all O2. Oxygen release rates below optimum may result in reduced aerobic metabolism or failure to maintain aerobic respiration. Of the oxidants tested, MgO2 has the widest application based on
- --O2 release rate, which was the longest
- --pH shift, which was lowest
- --O2 release per mass, which was highest
Oxygen release compound, commonly known as magnesium peroxide (MgO2), raises the dissolved oxygen concentration of aquifers, thereby creating conditions which may stimulate indigenous, petrophilic microbes to aerobically degrade petroleum contamination to carbon dioxide and water.
* Oxygen generation: Magnesium peroxide, when hydrated, releases oxygen per the following reaction: MgO2+H2O -> 1/2 O2+ Mg(OH)2
* Application methods: Magnesium peroxide can be introduced to an aquifer by either the retrievable filter sock method or by direct-push injection as a slurry. In situations where there is an open excavation as a result of either an underground storage tank removal, or remediation via excavation, magnesium peroxide as a dry powder can be mixed with low level contaminated soil before backfilling. Typically, the amount of compound applied to the soil is at least about 100 grams per metric ton of soil and preferably from about one to ten kilograms of compound per metric ton of soil.
* Uses: Some noteworthy uses of magnesium peroxide in the remediation of petroleum are:
(1)At sites where adequate nutrients for bioremediation already exist in an aquifer, and dissolved oxygen is all that is needed to accelerate the rate of contaminant biodegradation
(2)As an oxygen barrier for groundwater contamination plume control
(3)As a polishing step to meet target rehabilitation contaminant levels when active site remediation, such as pump-and-treat or other physical methods, is no longer cost-effective
(4) As the oxygen supplier, in combination with other injected bioremediation products that directly introduce either nutrients and/or microbes into aquifers
Bioremediation refers broadly to the use of microbiological populations to participate in the biodegradation, transformation or sequestration of a given environmental pollutant. A prerequisite for this process are the microbes found in the soil or groundwater that consume the harmful organic materials and remove them from the environment. The microbes leave behind carbon dioxide and water as decomposition products.
In-situ bioremediation is in-place bioremediation, (aerobic and anaerobic) without excavation of contaminated soil. Excess oxygen is required for an accelerated in situ bioremediation; this means that there must be aerobic conditions in the area to be cleaned up. The microbes will grow, reproduce and consume an ever increasing amount of harmful organic materials under aerobic conditions and with the optimal addition of nutrients.
When bioremediation is used, it is therefore essential to maintain aerobic conditions. The location to be cleaned up must be supplied with oxygen over a long period. Magnesium peroxide is primarily used as the main oxygen source for in situ bioremediation. The reason for this is related to the specific properties of magnesium peroxide. Magnesium peroxide in powdered form is stable over a long period. The total O2 release period will last from three months to one year, however, this is a site specific issue that is primarily dependent on contaminant load and groundwater velocity. Field experience has indicated that at most hydrocarbon contaminated groundwater sites magnesium compound continues to release oxygen for a period of at least 6 months' time.
Magnesium peroxide is a non-toxic compound with no potential adverse effects to the aquifer. The by-products of magnesium peroxide's reaction with water are oxygen and ordinary magnesium hydroxide, which is virtually insoluble. Thus, magnesium liberates only oxygen into the aquifer. The magnesium hydroxide is insoluble and remains as an inert faction of the soil or in the application of filter socks, the magnesium hydroxide is contained within the cloth and is removed from the well. It should be noted that magnesium peroxide and magnesium hydroxide are safe for human consumption as they are both used as anti-acids in common drug store products.
Magnesium peroxide is a fine, odorless and tasteless, white powder. When the pH shifts toward neutral, magnesium peroxide slowly releases oxygen via intermediate formation of hydrogen peroxide. Magnesium peroxide is primarily used as the main oxygen source for in-situ bioremediation. It also finds use in oxygenating the lower parts of artificial or natural lakes, as well as wastewater and effluent, in coating seeds to improve germination and seedling survival rates, in oxygenating the roots of plants, and as the bleach agent in personal formulations.
Calcium peroxide is an ecologically pure substance, which can be used in different fields of industry and agriculture.
In environmental protection it is used:
- -For treating waste water and remediation of groundwater
- -For decontaminating soil
In agriculture it is used:
- -As fertilizing rich with oxygen
- -For stimulating seed growth and their germinating power
- -For presowing treatment of rice seed, which allows to do planting not by seedlings, but by dry seeds, coated with calcium peroxide. Such a technique sufficiently decreases work expenditure and increases crop capacity.
In aquaculture it is used:
- -To provide sufficient dissolved oxygen
- -To adjust pH value
- -To reduce the subaqueous content of ammonium and nitrogen
- -To eliminate carbon dioxide and sulfureted hydrogen
- -To prevent anaerobe from proliferation and killing nosogenetic bacteria, defecating aqueous body
In poultry-raising it is used:
- -To decontamination of fodder
- -To increase productivity, hens safety and improving their eggs
In cattle-breeding it is used:
- -For prophylaxis of casein-stone formation in the abomasum and diarrhoea with newborn calves
- -As an antimicrobic effect
- -For stimulating protective organism strength
- -For normalizing activity of the alimentary canal
- -For activizing digestion work
- -For great increasing live-stock safety
In precious metal production it is used:
- -For leaching precious metals in the formation of cyano complexes (particularly complexes with gold and/or silver) from ores, ore concentrates, and other particle-shaped, solid materials.
In bakery industry it is used:
- -To improve bread crumb and its porosity
- -To keep moisture in dough during its baking
- -To initiate yeast growth
In dental care it is used:
- -For tooth bleaching
In the process for the melting of mineral compositions in preparation for their transformation into fibers e.g. glass, calcium peroxide is added to the batch of vitrifiable mineral components in the melting furnace to provide a low temperature oxidizing environment. The calcium peroxide may be added to the batch materials prior to their introduction into the furnace, or may be added directly to the furnace along with the mineral components. However, it is generally preferred that the calcium peroxide be premixed with the mineral components prior to their introduction into the furnace to ensure that the calcium peroxide is substantially homogeneously distributed throughout the composition. The calcium peroxide is preferably added to the composition in an amount up to about 5% by weight of the total composition.
As the temperature of the batch reaches about 575 - 600.degree. F. (301.7 - 315.6.degree. C.), the calcium peroxide begins to decompose into oxygen and calcium oxide. Decomposition of the calcium peroxide generally peaks at about 700.degree. F. (371.1.degree. C.). Accordingly, when the glass batch reaches such temperatures, oxygen is released from the calcium peroxide and creates an environment favorable for the oxidation of any organic impurities contained in the glass batch. Importantly, since the glass batch is still well below its melting point, the gaseous by-products of such oxidation, as well as any excess oxygen released from the calcium peroxide decomposition, are able to pass through the granular batch material and escape without forming an insulating layer that impedes melting of the batch. Further, by removing the organic impurities prior to melting of the mineral composition, the presence of carbon in the molten composition is reduced, which tends to reduce SO.sub.2 off gassing and to decrease the propensity for foam formation in the molten mineral composition.
As the temperature of the glass batch increases further within the furnace, the remaining calcium oxide dissolves into the molten glass. As a result, no residues are generated which undesirably affect the quality of the glass, nor are potentially environmentally unfriendly by-products generated by the decomposition of the calcium peroxide oxidizing agent.
Calcium peroxide is a yellowish solid peroxide which slowly decomposes to release oxygen at a "controlled" rate. It decomposes in moist air, is practically insoluble in water, and dissolves in acids, forming hydrogen peroxide. A 1:100 aqueous slurry has a pH of about 12.
Since its introduction in early 1989, there has been significant interest among the dental profession and the general public for home-use tooth bleaching products and methods. Typical dental bleaching compositions include from 5-20% by weight of carbamide peroxide (CO(NH2)2.H2O2), which is a complex of urea and hydrogen peroxide. However, sodium perborate has been found to be an another dental bleaching agent. An advantage of perborate-based bleaching agents rather than aqueous hydrogen peroxide or carbamide peroxide is that perborates are allowed for dental bleaching procedures in some countries that do not permit the use of aqueous hydrogen peroxide and carbamide peroxide for dental bleaching. Perhaps perborate compounds are more gentle on surrounding gums and tissues compared to either aqueous hydrogen peroxide or carbamide peroxide. Nevertheless, perborates were found to be unstable when blended with carboxypolymethylene, which is the tackifying agent of choice in the vast majority of home bleaching kits presently on the market. For this reason, a tackifying agent that is stable in the presence of perborate bleaching agents has been developed, which comprises a mixture of a suitable polyol and a finely divided gel-forming particulate such as fumed silica, otherwise known as silica fume.
Below is a sample dental bleaching composition that combines the following ingredients (in weight percent):
*Anhydrous Propylene Glycol 54.3%
*Fumed Silica 20%
*Sodium Perborate Monohydrate 25%
*Sodium Saccharine 0.7%
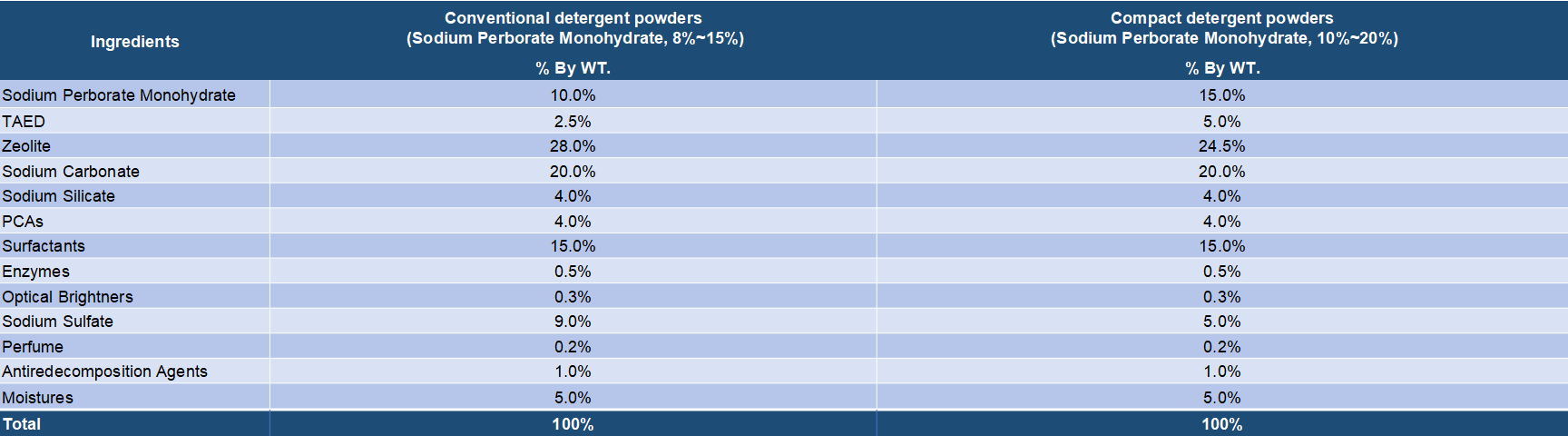
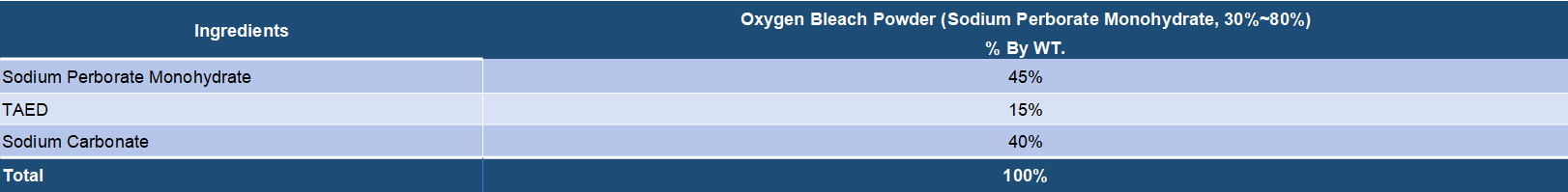
Both sodium perborate and sodium percarbonate are oxygen release bleaching chemicals that are widely applied in various bleach compositions. It is known that sodium perborate as the bleaching agent has a high bleaching effect at high temperatures but the effect is lowered at low temperatures. On the other hand, sodium percarbonate has an effective bleaching action even at low temperatures and is very valuable from the viewpoint of saving of energy. Sodium percarbonate is an attractive perhydrate for use in detergent compositions because it dissolves readily in water, is weight efficient and, after giving up its available oxygen, provides a useful source of carbonate ions for detergency purposes. Sodium serborate has a better stability and has been a mature bleaching ingredients for long time. But it is increasingly replaced by sodium percarbonate duo to its disadvantages in energy saving and environment protection. Sodium percarbonate exhibits an excellent bleaching effect even at a low temperature and is environmentally friendly, but it is less stable in detergent formulations. However, many processes have been found to improve its stability.
Sodium perborate usually exists in two forms, tetrahydrated and monohydrated. Sodium perborate tetrahydrate is obtained by addition of hydrogen peroxide to a sodium metaborate solution at a temperature close to 20.degree. C. Sodium perborate monohydrate is produced by dehydrating sodium perborate tetrahydrate in a fluid bed with heated air. Sodium perborate releases nascent oxygen at elevated temperatures, and so acts as a hydrogen peroxide bleach. The monohydrated form is essentially showing three advantages in comparison with the tetrahydrated form: a higher content of available oxygen, a higher heat stability and a higher dissolution rate into water. Sodium perborate has been in detergent and personal care formulations for many years. Its oxidative power improves the cleaning, bleaching, stain removal and deodorizing performance of powder detergent formulations, all fabric dry bleachs, denture cleaners, automatic dishwasher detergents and various institutional and industrial laundry products. It's main disadvantage is that the bleaching action only takes place at elevated temperatures. To release it's bleaching action at lower temperatures, an activator must be added.
--Calcium peroxide facilitates to remove the oceanic or lacustrine red tides. By adding 100mg~500mg of calcium peroxide per Liter of water, the occurrence of red tide can be eliminated in 24 hours.
--By coating rice seeding with calcium peroxide, herbicide and plaster of paris, usually 4 kgs calcium peroxide for 24 kgs of rice seeds, yield can be increased by 10% and the cost can be reduced by 50%.
--The harmful CO (carbon oxide) from the lighted cigarette may be greatly reduced when calcium peroxide (amount 0.5%~10%) is added during the manufacturing process. At the smoking rate of 50ml/s, the CO discharging amount of the calcium peroxide blended is remarkably reduced to 2700mg/kg, as compared with the amount of 5000mg/kg discharged by normal cigarette.
--Calcium peroxide is administered to the pigs via the oral route in a proportion of 0.02 to 1.3 % by weight of the total food ration. The percentage of lean meat and the quality of the carcasses are thus substantially improved.
--Calcium peroxide may be used in the detoxification of waste water containing cyanides and/or cyano complexes, heavy metals and sulfides with a substantial effect.
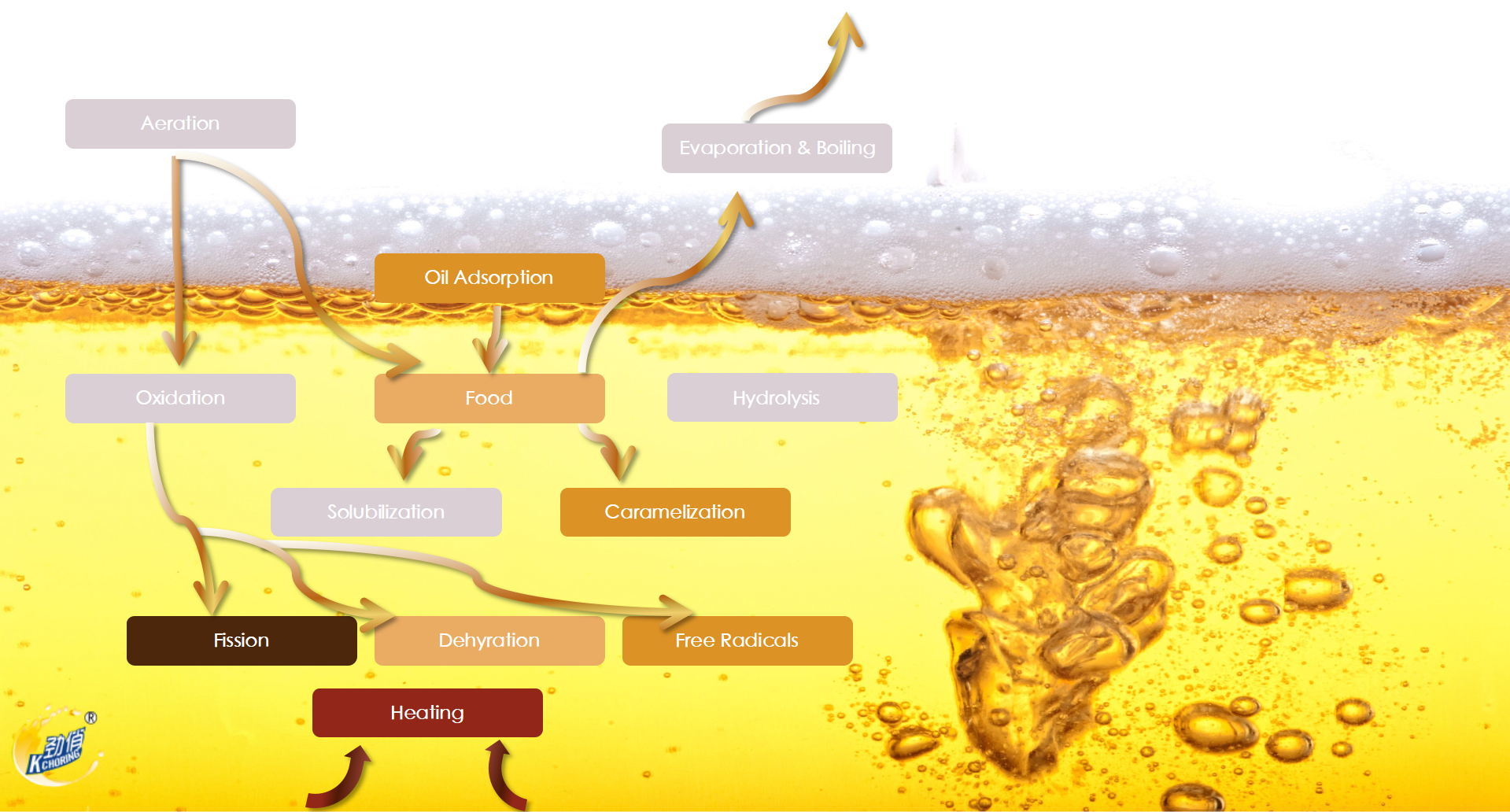
Six Enemies of Frying Oil:
Air:Air bubbles that are pumped into oil during filtering causes oxidation and impacts fried food flavor.
Heat:If the fryer's temperature is too high, the oil oxidation will happen rapidly.
Soap:Grease cleaning can produce an alkaline flavor and causes oil to darken.
Carbon:Bread Crumbs and small pieces of food will carbonize and cause chemical reactions, resulting in oil breakdown.
Water:Water from food, steam, and residual water after cleaning will cause hydrolysis, which will cause oil to darken and smoke.
Salt:Salt exists in marinades and breading breaks will cause solubilization, Which triggers a chemical reaction that begins the breakdown and reduces the service life of the oil.
The main reactions during the use of frying oil include starch gelatinization, Maillard reaction, protein denaturation, caramelization reaction, etc. Unsaturated fatty acids are oxidized to peroxides and hydroperoxides, which continue to decompose into fatty acids, aldehydes, ketones, and other small molecules, and are finally oxidized to acids. As a result, the oil deteriorates, increasing the acid value, peroxide value, and viscosity of the frying oil, darkening the color of the oil, increasing the trans fatty acids, aggravating the foam of the oil, and affecting the smell and taste.
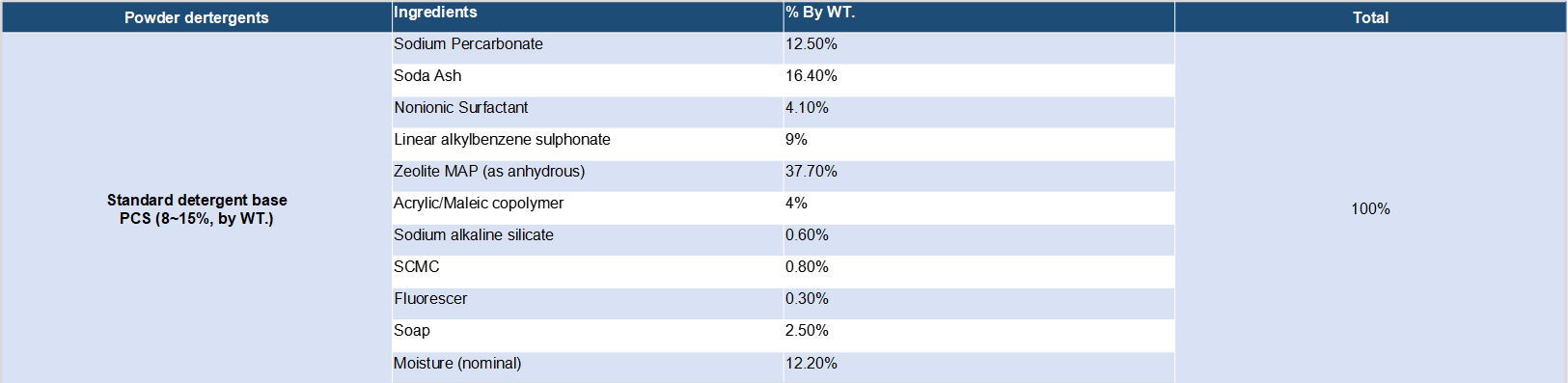
Detergent compositions containing sodium percarbonate are known in the art. Sodium percarbonate is an attractive perhydrate for use in detergent compositions because it dissolves readily in water, is weight efficient and, after giving up its available oxygen, provides a useful source of carbonate ions for detergency purposes. However, the inclusion of percarbonate salts in detergent compositions has been restricted by the relative instability of the bleach both as is and in use. Sodium percarbonate loses its available oxygen at a significant rate in the presence of ions of heavy metals such as iron, copper and manganese and also in the presence of moisture, these effects being accelerated at temperatures in excess of about 30.degree. C. To solve this problem, several solutions have been found.
1. Sodium percarbonate is coated with a hydrophobic substance or the like.
2. Magnesium silicate is incorporated in a detergent composition containing sodium percarbonate.
3. A chelating agent which forms an easily water-soluble metal chelated compound such as nitrilotriacetate (NTA) or ethylene diamine tetraacetate (EDTA) is incorporated in a detergent composition.
4. Zeolite A is replaced by maximum aluminium zeolite P (zeolite MAP) since zeolite MAP itself is of greater liquid carrying capacity than zeolite A.
5. The elimination of impurities, such as heavy metals which catalyze the decomposition reaction during detergent processing, alleviates the instability of aqueous SCP solutions.
6. Provide sufficient sodium carbonate in the composition to be able to combine with all of the available water in the composition to form sodium carbonate monohydrate, the term "available water" includes water chemically available as hydrogen peroxide, water of crystallization of sodium carbonate hydrates and free water which may temporarily exist in the composition.
Sodium percarbonate is used as an active oxygen component in detergents, bleaches and cleaning agents. Due to the unsatisfactory storage stability of the uncoated sodium percarbonate in warm/moist surroundings and in the presence of certain detergent and cleaning agent components, sodium percarbonate must be stabilized against the loss of active oxygen. An essential principle of stabilization involves encasing the sodium percarbonate particles in a coating of components having a stabilizing action. Here comes the definetion: the coated sodium percarbonate is the sodium percarbonate crystals coated with single or multiple layers of various substances in order to increase active oxygen stability and optimize storage and ensiling properties.
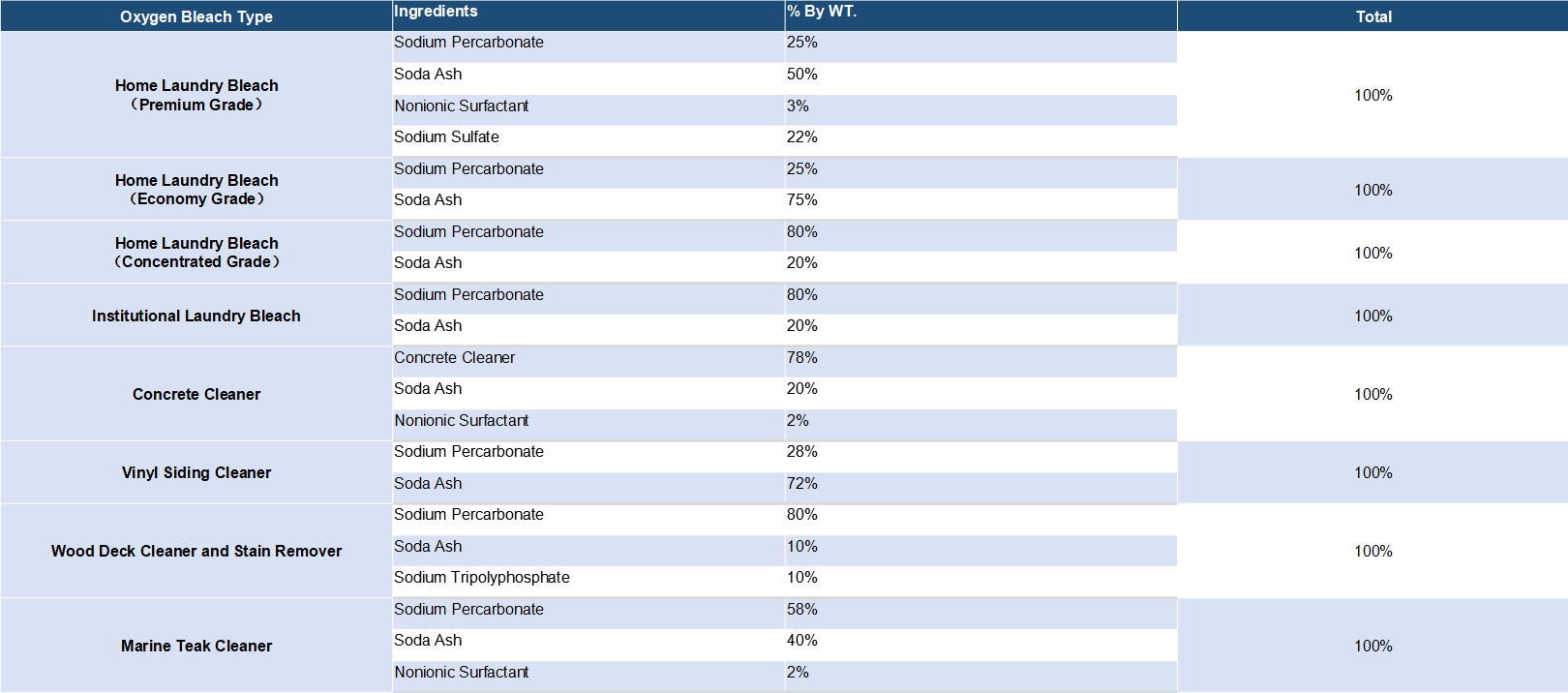
Coated sodium percarbonate is the more commonly commercialized peroxide compared with the uncoated sodium percarbonate. But the uncoated product is still the preferred ingredient for simply mixing with enough quantity of soda ash and some surfactants to form the popular oxygen bleaches.
JHY production is located in Shangyu, Zhejiang, which has been producing sodium percarbonate for more than a decade. Due to its abundant R&D investment and tech innovation, JHY can provide its customers with different specs of quality coated and uncoated sodium percarbonate. We currently have a production capability of 30,000t annually which can meet customers' increasing demands on a sustainable base. You'll feel satisfied with the cooperation with JHY not only for the reason of good product quality, timely and safe delivery, but also for the excellent after-market service and tech support. JHY runs its business on the base of honesty and integrity, We fully participated in meeting customers' demands.








