|  |
JHY Services & Support
FAQs & Tips
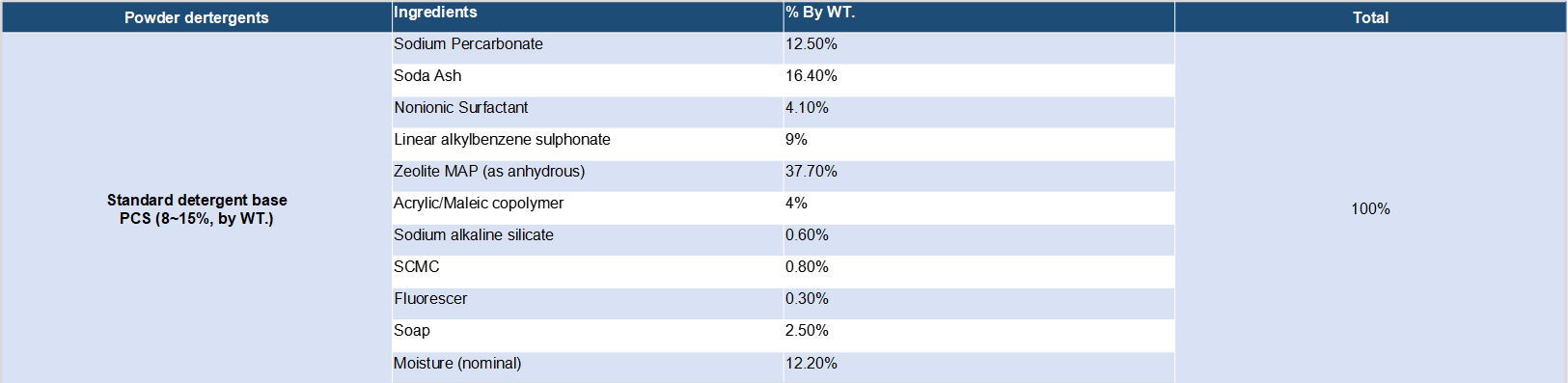
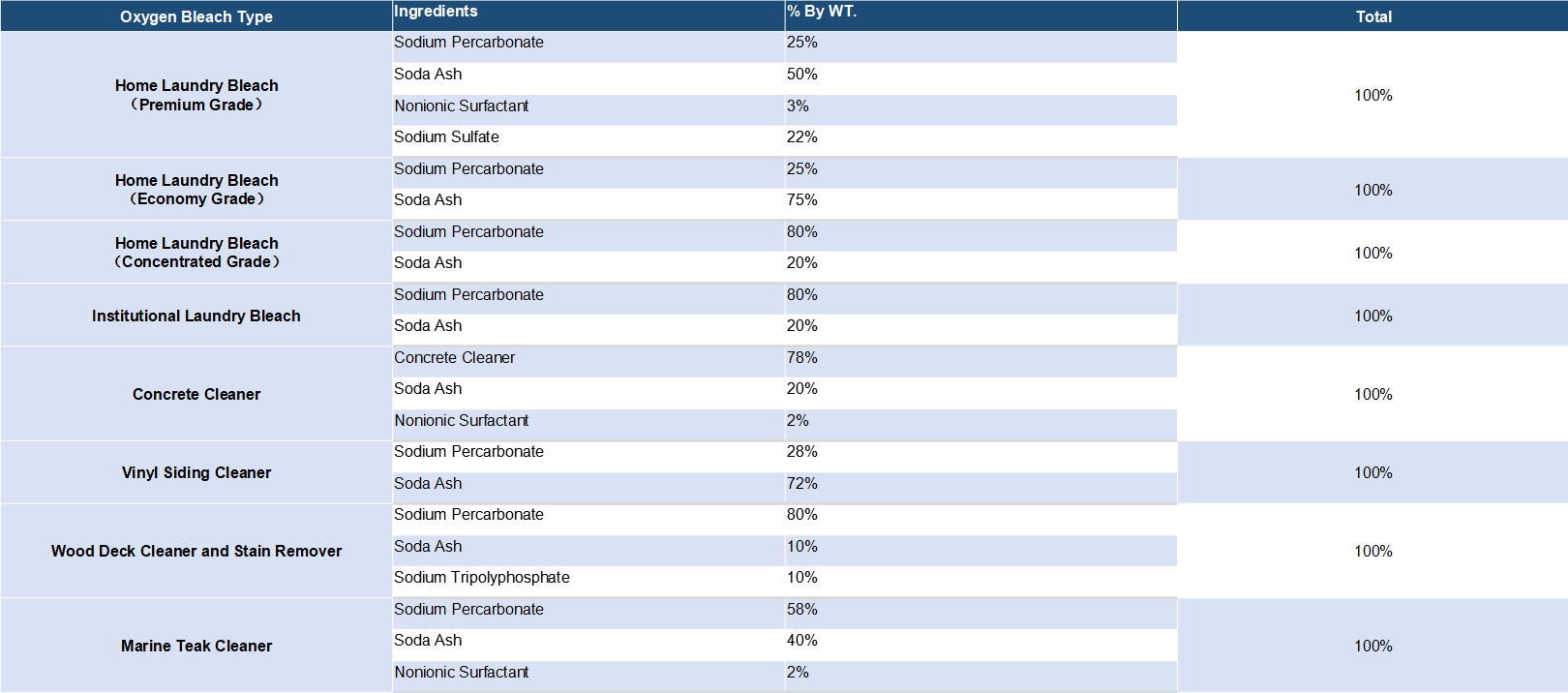
Detergent compositions containing sodium percarbonate are known in the art. Sodium percarbonate is an attractive perhydrate for use in detergent compositions because it dissolves readily in water, is weight efficient and, after giving up its available oxygen, provides a useful source of carbonate ions for detergency purposes. However, the inclusion of percarbonate salts in detergent compositions has been restricted by the relative instability of the bleach both as is and in use. Sodium percarbonate loses its available oxygen at a significant rate in the presence of ions of heavy metals such as iron, copper and manganese and also in the presence of moisture, these effects being accelerated at temperatures in excess of about 30.degree. C. To solve this problem, several solutions have been found.
1. Sodium percarbonate is coated with a hydrophobic substance or the like.
2. Magnesium silicate is incorporated in a detergent composition containing sodium percarbonate.
3. A chelating agent which forms an easily water-soluble metal chelated compound such as nitrilotriacetate (NTA) or ethylene diamine tetraacetate (EDTA) is incorporated in a detergent composition.
4. Zeolite A is replaced by maximum aluminium zeolite P (zeolite MAP) since zeolite MAP itself is of greater liquid carrying capacity than zeolite A.
5. The elimination of impurities, such as heavy metals which catalyze the decomposition reaction during detergent processing, alleviates the instability of aqueous SCP solutions.
6. Provide sufficient sodium carbonate in the composition to be able to combine with all of the available water in the composition to form sodium carbonate monohydrate, the term "available water" includes water chemically available as hydrogen peroxide, water of crystallization of sodium carbonate hydrates and free water which may temporarily exist in the composition.
Sodium percarbonate is used as an active oxygen component in detergents, bleaches and cleaning agents. Due to the unsatisfactory storage stability of the uncoated sodium percarbonate in warm/moist surroundings and in the presence of certain detergent and cleaning agent components, sodium percarbonate must be stabilized against the loss of active oxygen. An essential principle of stabilization involves encasing the sodium percarbonate particles in a coating of components having a stabilizing action. Here comes the definetion: the coated sodium percarbonate is the sodium percarbonate crystals coated with single or multiple layers of various substances in order to increase active oxygen stability and optimize storage and ensiling properties.
Coated sodium percarbonate is the more commonly commercialized peroxide compared with the uncoated sodium percarbonate. But the uncoated product is still the preferred ingredient for simply mixing with enough quantity of soda ash and some surfactants to form the popular oxygen bleaches.
JHY production is located in Shangyu, Zhejiang, which has been producing sodium percarbonate for more than a decade. Due to its abundant R&D investment and tech innovation, JHY can provide its customers with different specs of quality coated and uncoated sodium percarbonate. We currently have a production capability of 30,000t annually which can meet customers' increasing demands on a sustainable base. You'll feel satisfied with the cooperation with JHY not only for the reason of good product quality, timely and safe delivery, but also for the excellent after-market service and tech support. JHY runs its business on the base of honesty and integrity, We fully participated in meeting customers' demands.
* Compared with chlorine bleaching chemicals that have contaminations on the environment, sodium percarbonate is an environmentally friendly chemical which decomposes into oxygen, water and natural soda ash when in contact with hydrous media.
* Sodium percarbonate is increasingly being the substitute for sodium perborate in detergent formulations due to its lower dissolving temperature in water, as well as the characteristic of no contamination on soil, as sodium perborate is made of borax which is found to have negative impact on the soil quality.
* Detergent or bleach compositions formulated with sodium percarbonate have an strong stain removal capability. It is very effective as a laundry presoak for heavily stained articles. It is color safe. It brightens colors and prevent fabric form become yellowed or darkened.
* Sodium percarbonate is effective as a disinfectant on both bacteria and virus. It's an excellent ingredient in personal care and home care formulations for hygiene.
* For its environmental advantages, sodium percarbonate is a good oxygen release chemical for agricultural and aquicultural applications.
Sodium percrabonate has a wide range of applications in various solid detergent products and all fabric bleaches. We may also find its usages in oxygen release compositions, personal care formulations, disinfectants, food bleaches, pulp and paper bleaches and textile bleaches, etc.
- Sodium percarbonate (or sodium carbonate peroxyhydrate) is an addition compound of sodium carbonate and hydrogen peroxide. When dissolved into water, its releases H2O2 and soda ash (sodium carbonate).
- The type of reaction shown as follows: 2Na2CO3.3H2O2 → 2Na2CO3+ 3H2O2
- The pH of the resulting solution is typically alkaline, which activates the H2O2 for bleaching. The dry powder contains about 30% w/w H2O2.
Sodium percarbonate is known for being non-corrosive and safe to use on most indoor or outdoor surfaces except finished and unfinished wood surfaces. So it is important to note that sodium percarbonate should not be use on hardwood floors.
Sodium percarbonate should be stored in a sealed container at room temperature. This chemical, similar to solid hydrogen peroxide, can be irritating and corrosive to the skin and especially to the eyes. Therefore, it is crucial to keep it out of the reach of children. Ingestion should be strictly avoided at all concentrations, and we recommend using gloves when handling it for cleaning applications. Sodium percarbonate is known to be human-safe and biodegradable, making it generally safe for disposal in the trash. However, we advise checking with local regulations to ensure proper disposal methods.
Yes and no.
Since sodium percarbonate is a natural bleaching agent, it may discolor your clothes if left too long or used undiluted or in high concentrations. However, when diluted according to package directions, JHY sodium percarbonate can be safely used on white or colored clothing with minimal risk of discoloration. We recommend conducting a small patch test to check how your clothing reacts before full use.
Sodium percarbonate may begin to decompose after one year. However, this does not necessarily mean that it won't work or is dangerous to use. It may simply lose some effectiveness after one year.








